9ft of snow?! I only experienced such deep snow in an urban setting while living in Connecticut for a year. I spent a few years in Oregon but the snow in the area never got so deep while I was there. When I was in the US I was not yet able to identify many fungi as I was mainly obsessed with animals (especially salamanders) back then, so unfortunately I did not really appreciate the diversity of fungi there. Although once in Oregon I did attempt to dye some socks using a wolf lichen (Letharia vulpina) and a pressure cooker. That did not end well.
Sal
I see. So it is not necessarily that their mycelium are better at surviving the freezing temperatures, but rather that either they fruit quicker once conditions are acceptable or that their fruiting bodies are more cold tolerant. Thanks, it's interesting.
Cool! I just read their wiki page and it says
A snowbank fungus, it is most common at higher elevations after snowmelt in the spring.
Snowbank fungus is a new term for me. Not sure yet what makes a fungus thrive through snow. Maybe they have anti-freeze proteins?
Does your area get a lot of snow?
In many cases the tweet is important because it was tweeted by a specific person. If only a screenshot is provided then one needs to go through the hassle of verifying it is a real tweet. A good approach would be to do both: post screenshot + link to neutral frontend
Congrats!! Is your trip still ongoing, or you are done for now?
Alright! Some other tips:
- Your current microscope is a 160 mm system, so make sure that the objectives are 160 mm and not infinity.
- Make sure the objectives have an RMS thread
- Once you move into higher-end objectives, you will have objectives that are specialized. For example, 'phase contrast' objectives have a dark ring inside of them. For the olympus brand their name often ends in 'PL'. These work with bright-field too. My 40/1.30 objective is actually a phase-contrast objective because I did not know this and ChatGPT told me it meant something different 😂 However, the objective does work well for me and I am now considering upgrading to a phase contrast-capable microscope (the BH2), so I made a good choice by accident.
I first purchased some Plan objectives from China (40x, 60x), and they are alright. More recently I have been looking into objectives with high numerical apertures to increase the resolution of my images, and I think that the best source of good high-quality used objectives is Ebay. The Olympus apochromatic objectives with high NA are listed for a fraction of their original price, but they are still in the $200 - $500 range, so not very cheap.
Wow, those spores are so bumpy, they are very interesting! Thanks for sharing :D
Ah, I did miss this one! I am not sure that I was notified. This one would absolutely fit with the general theme, as it is a community about sharing useful math-based perspectives.
Thanks for the idea! That looks very nice, I like how the collection is organized in those storage containers. They look very well preserved so far so your well water + sun protocol seems to be working well. Perhaps I will too start a lichen collection!
























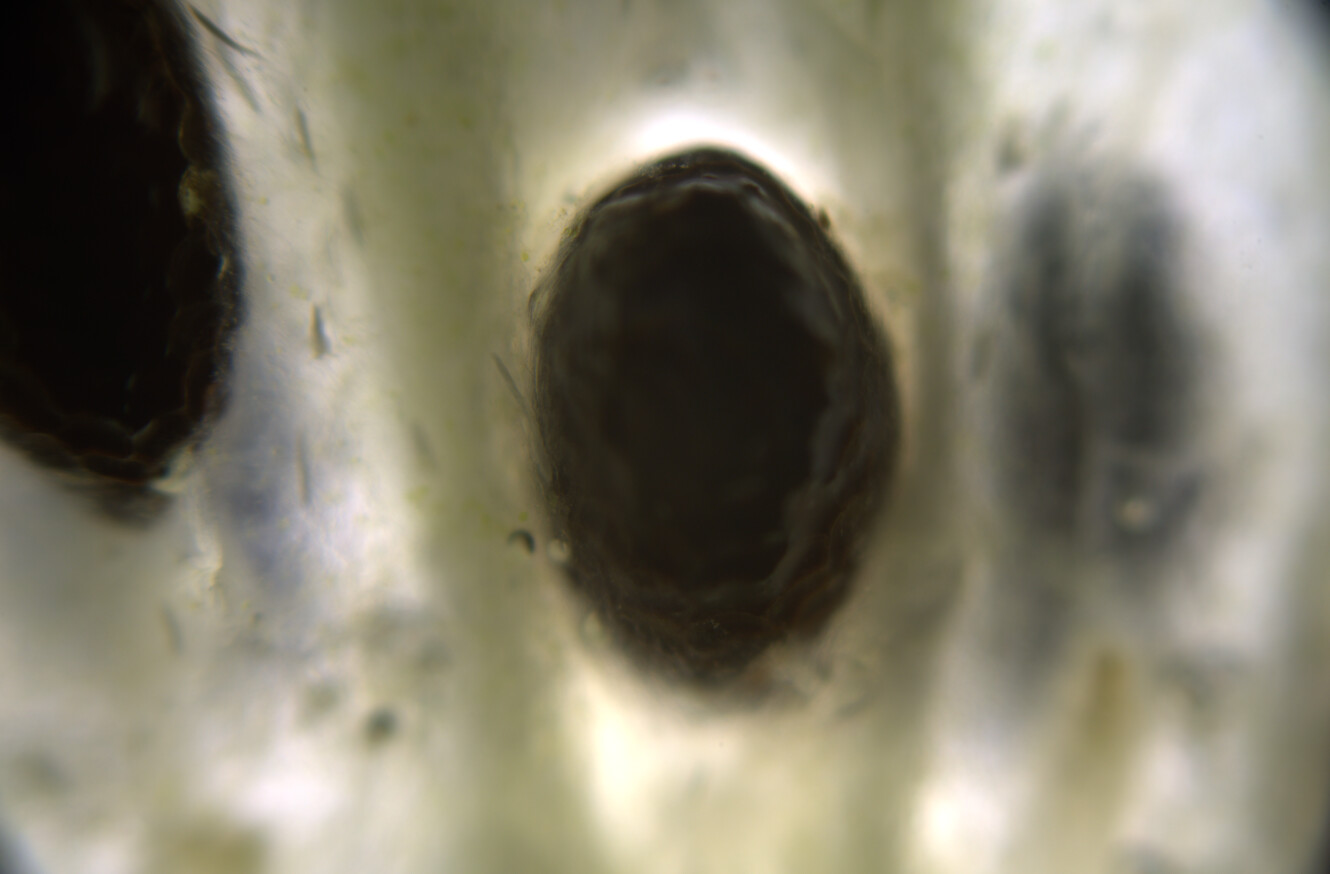

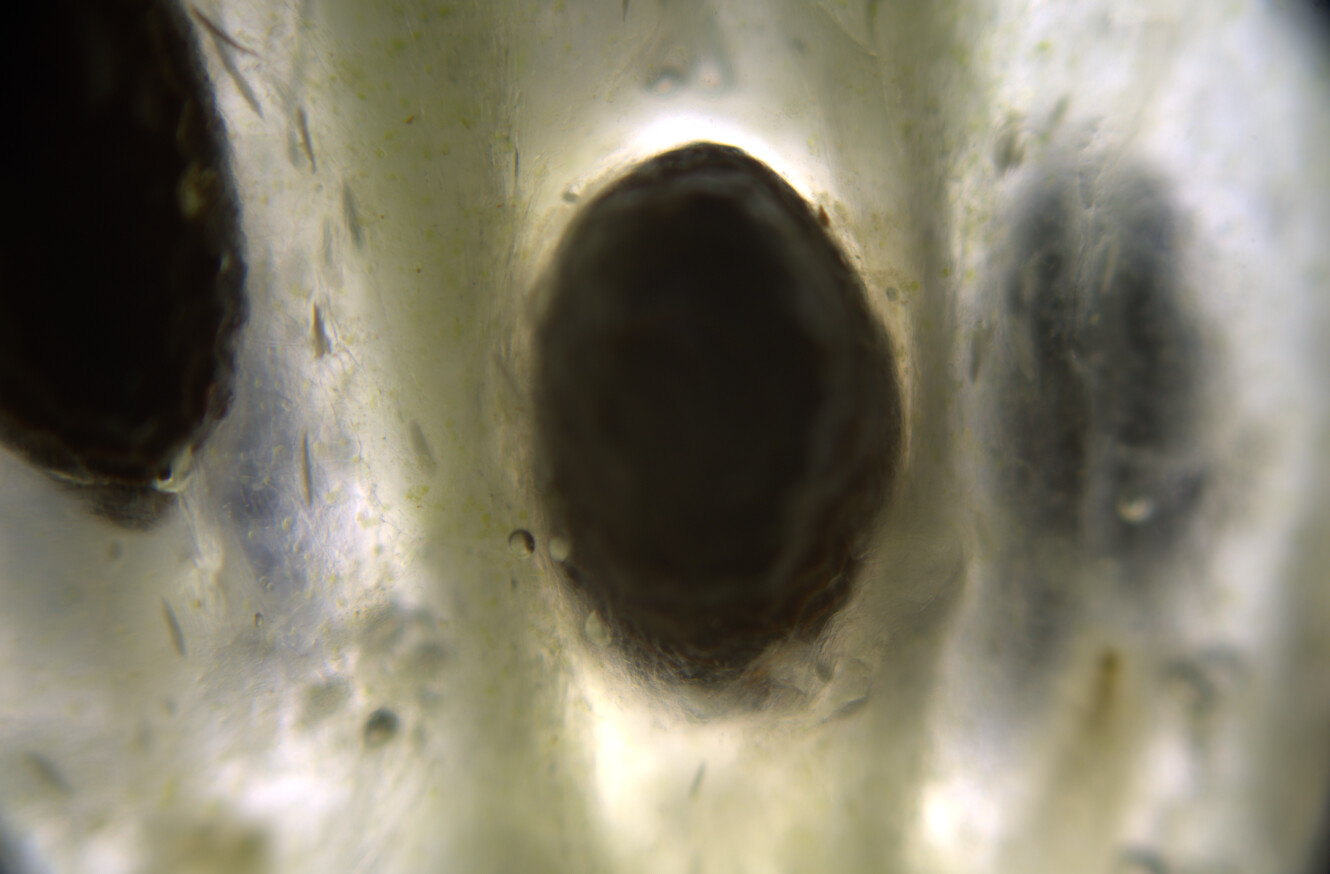
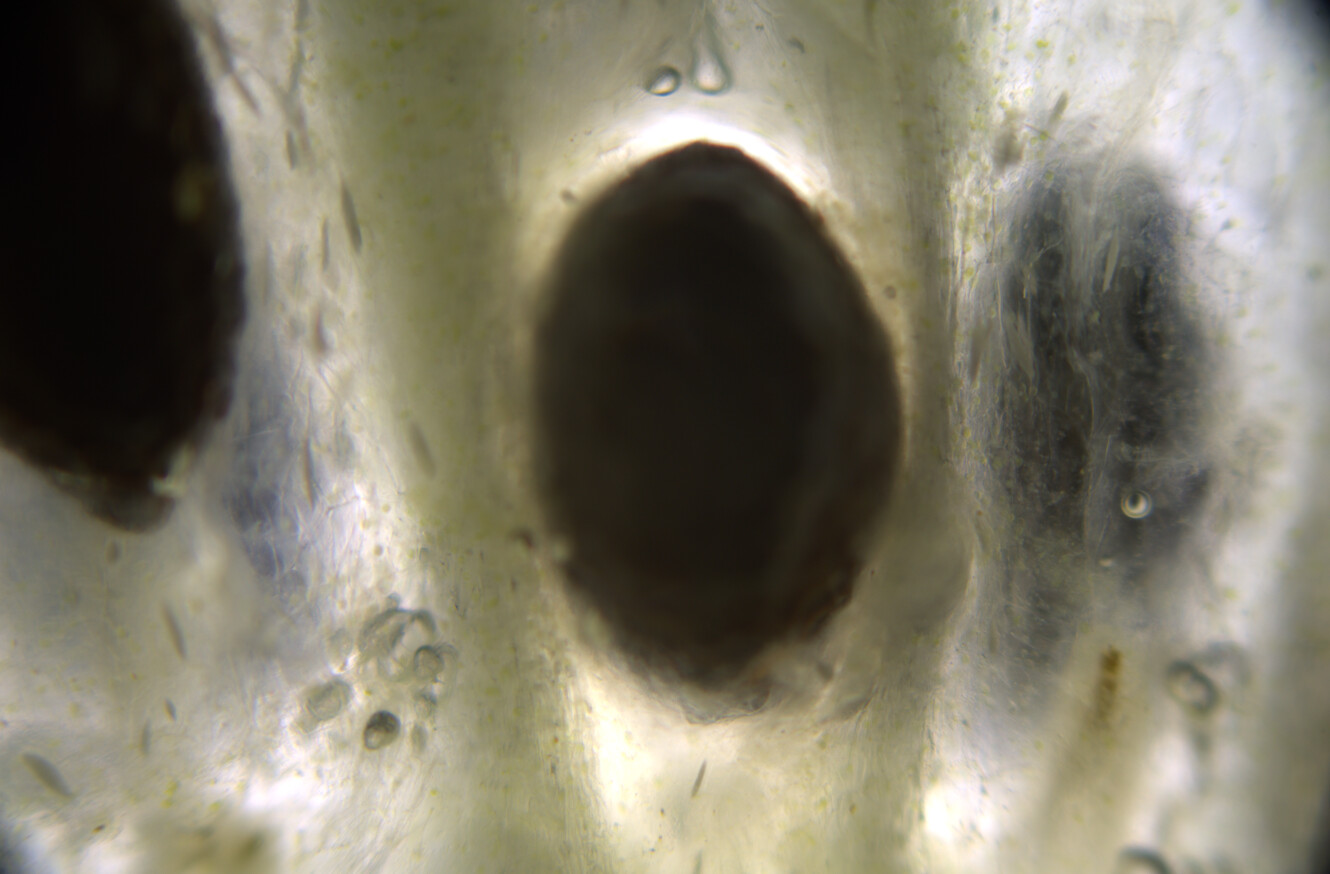









Would love to... When I was in Oregon this lichen was super abundant. At the moment I am living in Amsterdam (Netherlands), and I see mostly Xanthoria, Evernia, Rhizocarpon, and a few other lichen species that grow on city trees, but they are very small and spotty, nothing compared to the wolf lichen in Oregon. I do miss the Oregon forests with the old growth sequoia redwood trees and all that lichen.